Abstract
Background: Chimeric Antigen Receptor (CAR) T cells therapy has shown remarkable success in haematological malignancies. However, complexity of the manufacturing platform and exorbitant costs of the available therapy limits their accessibility in limited resource settings including India. Recently, we developed a novel humanized anti-CD19 CAR-T cell therapy (HCAR19) and showed its efficacy in preclinical models (Dwivedi et al, Mol Cancer Ther. 2021). Here we report our experience of HCAR19 manufacturing in an integrated platform for first-in-human Phase-I clinical trial. Further, we performed the cost analysis of CAR-T delivery from development to clinic.
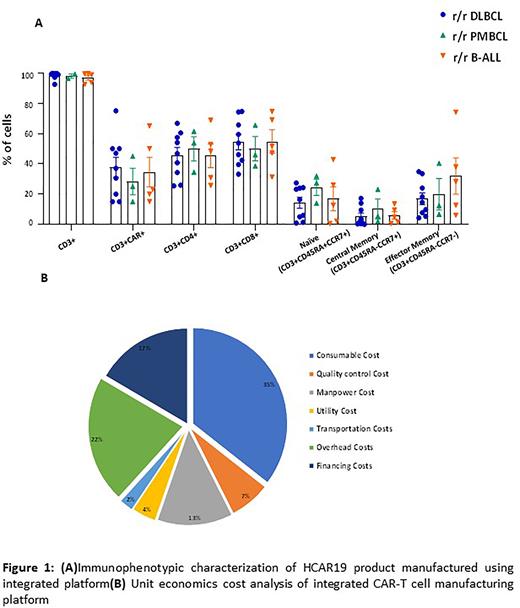
Methods: Lymphocytes were collected from 17 patients of r/r B cell malignancies (DLBCL, transformed follicular lymphoma, PMBCL and B-ALL) through apheresis (Fresenius Fomtek or Spectra Optia) upon receiving the regulatory approvals from central and local agencies. CD3+ T cells were enriched and activated using CTS™Dynabeads™ CD3/CD28 for 36-48 hours followed by consecutive double transduction with CD19 encoding lentivirus vector. Final product was harvested, cryopreserved and stored in ultra-low temperature until further use. All steps were carried out in closed gas permeable bags. Final product quality control (QC) assays were performed by flowcytometry, ELISA, analytical and cell-based assays. The cost of CAR T cell manufacturing was calculated for unit economics on fully scale production model by standard costing methodology. The final unit economics included cost of goods and services (COGS), overhead costs, financing costs of infrastructure, R&D and clinical trials costs. The model was scaled towards a 200 patient per annum in a centralized manufacturing facility.
Results: Of the 19 patients enrolled in the study, only 17 patients underwent apheresis procedure for harvest of total 5-10 X 109 mononuclear cells. HCAR19 cells from all 17 patients were manufactured successfully in functionally closed integrated manufacturing platform. The required HCAR19 dose was achieved within 8 days of culture (dosage range: 1.78 x 106 - 17 x 106 viable HCAR19/kg). The transduction efficiency of HCAR19 in r/r DLBCL, r/r PMBCL and r/r B-ALL was 37.7 ± 6.64%, 28.8 ± 8.81% and 34.6 ± 9.72% (Mean ± SEM) respectively . The HCAR19 showed a robust expansion (~20-fold) ex vivo and maintained the presence of potent anti-tumor subsets like naïve and central memory T cells (Figure 1a). Final product from all 17 patients qualified the QC release criteria for safety, identity, purity and potency as per specifications. Finally, the unit economics in fully-scaled production model indicated the estimated cost of CAR-T cell delivery was approximately 30,000 USD (or 25,00,000 INR) per dosage including logistics, lentiviral vector cost and quality control (Figure 1b). The major costs incurred were towards consumables, overhead and financing costs of clinical trials and infrastructure. There was very minor components of logistics and utility costs. More importantly, the major contributors of overall low cost of CAR-T delivery in our settings were frugal R&D costs in an academic setting, scalable integrated manufacturing platform, and highly qualified yet economical manpower.
Conclusions: We developed a robust, scalable and highly affordable CAR-T cell therapy platform (product and process development, and first-in-human clinical study), and proven its clinical delivery successfully with our first indigenous HCAR19 product. Given the benefit of affordable platform to broader socio-economic base of patients, the cell and gene therapy will be poised for a transformational change as a mass therapy.
Disclosures
Karulkar:Immunoadoptive Cell Therapy Pvt Ltd: Current Employment. Shah:ImmunoAdaptive Therapy Pvt Ltd: Current Employment. Firfiray:Immunoadoptive Cell Therapy Pvt Ltd: Current Employment. Ravikumar:ImmunoAdoptive Therapy Pvt Ltd: Current Employment. Purwar:Immunoadoptive Cell Therapy Pvt Ltd: Current Employment, Current equity holder in private company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal